Stimulated Emission Depletion Fluorescence Microscopy (STED)
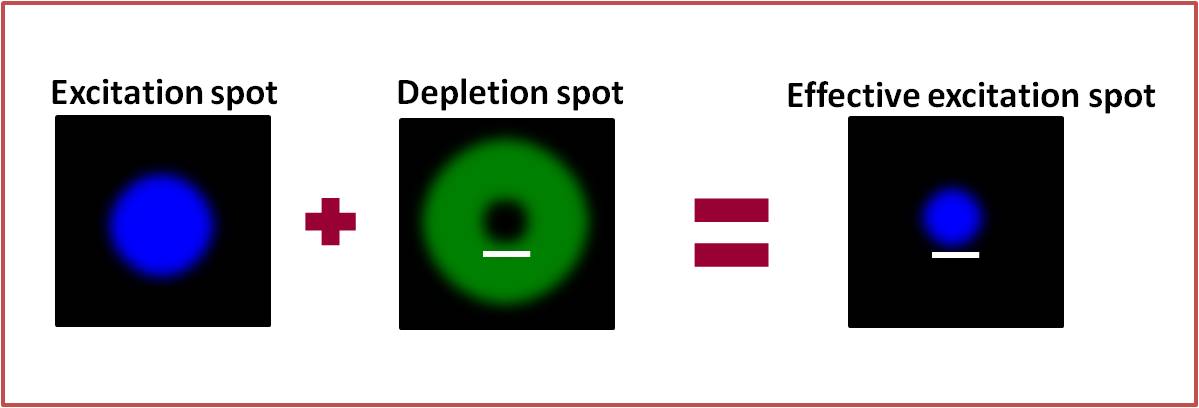
Fluorescence microscopy has given fundamental insights into the study of sub-cellular architecture and dynamics but many details remain unresolved due to the diffraction limit inherent to light microscopy. STED microscopy, first suggested by Stefan Hell in 1994 [1] was the first fluorescence microscopy technique to deliver super-resolution images that successfully surpassed the diffraction barrier. The central idea behind STED is to spatially minimize the effective focal spot created by a focused laser, thereby increasing the resolution of confocal fluorescence microscopy. This is accomplished using a doughnut shaped, red-shifted depletion beam that is focused and superimposed onto the excitation beam so that it can deplete fluorescence from the periphery of the diffraction limited excitation region. The depletion beam quenches fluorescence soon after the excitation of the fluorophore through the process of stimulated emission resulting in a sub-diffraction emission spot in the centre. The doughnut shape of the depletion beam is made possible through the use of a vortex phase plate, which causes a helical phase shift in the beam path, the outcome of which is a zero intensity central point. Now that STED has been around for over a decade, many advances have been made in this technique, such as dual colour STED [2] and live cell imaging [3]. Furthermore, the ease with which it can be coupled with techniques such as Fluorescence Correlation Spectroscopy [4] has made it especially popular in the life sciences. For a complete review of STED microscopy, please refer to the paper by Müller, T. et al. [5].
